How Glowing Patches Work: A Look at Phosphorescent Threads
in Custom Patches, Glow in the Dark Patches, Phosphorescent ThreadsGlow-in-the-dark patches work through phosphorescent threads, typically made from materials like zinc sulfide and strontium aluminate. These materials absorb light energy, especially from UV sources, and then gradually release it, creating that glowing effect. When you expose these patches to bright light, electrons within the materials get excited and store energy. They release this energy slowly when it’s dark, enabling the glow. Various factors affect the intensity and duration of the glow, so make sure to check product details for quality. Understanding these mechanics is essential if you want ideal performance from glow-in-the-dark items.
What Are Phosphorescent Threads?
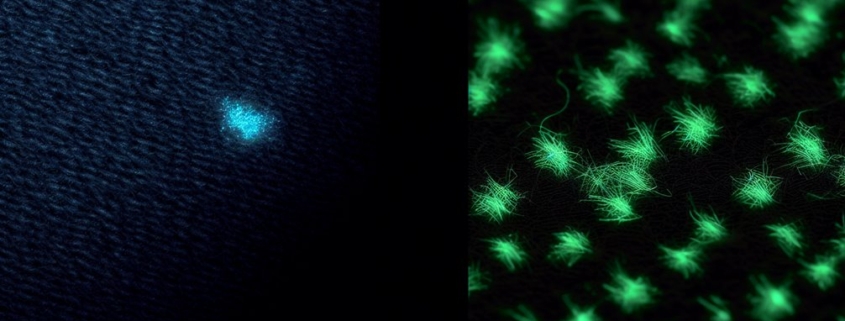
Phosphorescent threads are the secret behind those enchanting glow-in-the-dark patches you see on clothing and gear. These innovative materials harness the unique properties of phosphorescent materials, which absorb light and then release it slowly over time. When you expose these threads to a light source, they soak up energy, storing it like a battery. Once the lights go out, the stored energy is gradually released, creating the mesmerizing glow you admire.
What Are the Material Properties of Glowing Patches?
The glow properties of phosphorescent threads depend on the specific materials used in their composition. Common phosphorescent materials include zinc sulfide and strontium aluminate, both known for their ability to emit light for extended periods. However, not all phosphorescent threads are created equal; some may glow brightly but for a shorter duration, while others may emit a softer light for longer.
The Science of Light Absorption
Light absorption is a fascinating process that plays an integral role in how glow-in-the-dark patches function. When you expose these patches to light, they interact with light properties in remarkable ways. The threads contain phosphorescent materials, which are specifically designed to absorb photons—the tiny packets of light energy. This interaction is essential, as photon absorption allows the materials to store energy temporarily.
As the light hits the patch, its energy excites the electrons in the phosphorescent threads, pushing them to a higher energy state. Think of it like charging a battery. However, this process doesn’t happen without limitations. Not all light is absorbed equally; certain wavelengths are more effective than others. For example, ultraviolet (UV) light is particularly efficient at charging these patches, while visible light might not charge them as effectively.
Understanding light absorption is critical, especially when using glow-in-the-dark patches. If you want them to glow brightly and for an extended period, you need to guarantee they’re exposed to the right kind of light for adequate photon absorption. Otherwise, their effectiveness diminishes, and you won’t achieve the glow you expect.
Applications of Glow-in-the-Dark Patches
Glow-in-the-dark patches have found a variety of innovative applications across different fields. You might be surprised to see how these patches blend fashion trends with vital safety features. For instance, they’re commonly used in children’s clothing, helping parents spot their kids in low-light situations. In sportswear, these patches enhance visibility during evening runs, ensuring that athletes stay safe.
Here’s a closer look at some key applications:
Application Area | Example Use | Benefits |
Fashion | Glow-in-the-dark apparel | Trendy and eye-catching designs |
Safety Gear | Work uniforms | Increased visibility for workers |
Home Decor | Wall stickers | Unique nighttime aesthetics |
Emergency Equipment | First aid kits | Quick identification in the dark |
Personal Accessories | Bags and backpacks | Added safety for night outings |
As you can see, glow-in-the-dark patches aren’t just fun; they serve vital roles in safety and style. Whether you’re dressing up for a party or ensuring your child’s safety, these patches offer practical solutions and keep you informed about potential hazards.



Leave a Reply
Want to join the discussion?Feel free to contribute!